GDPR-Compliant Bioinformatics by Design
Patient rights and data integrity as defaults in an experimental analysis portal
Every biotech executive has the same nightmare: a data breach halts your trial, exposes your critical IP, and scares off future investors. GDPR exists to prevent this nightmare; in short, it is Europe’s strict privacy rulebook (but, unlike most lab SOPs, this one comes with fines up to €20 million when things go wrong).1
Despite its reputation in biotech as a bureaucratic hurdle, GDPR reflects principles that not only protect data, but in practice, handle it to an end that supports the reproducibility and validation of experimental output for clinical translation.
For solution providers, GDPR’s value is clearer; it presents a commercial advantage and aligns your analysis with the same values that patients, clinicians, regulators, and software solutions already demand: accuracy, transparency, and accountability. It creates a common standard upon which geographically-diverse client data moves seamlessly from research to clinic, across international borders, and through tools without losing integrity along the way.
This post will apply key GDPR principles to the lab, operationalizing them with LatchBio’s approach to default-compliant product development.
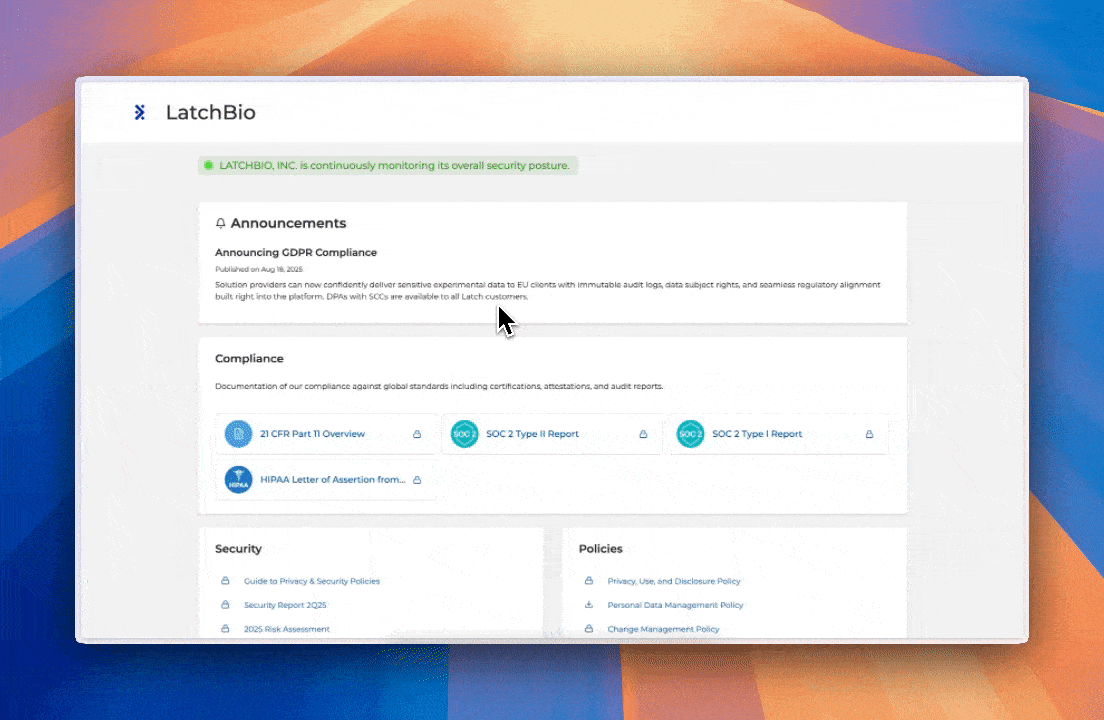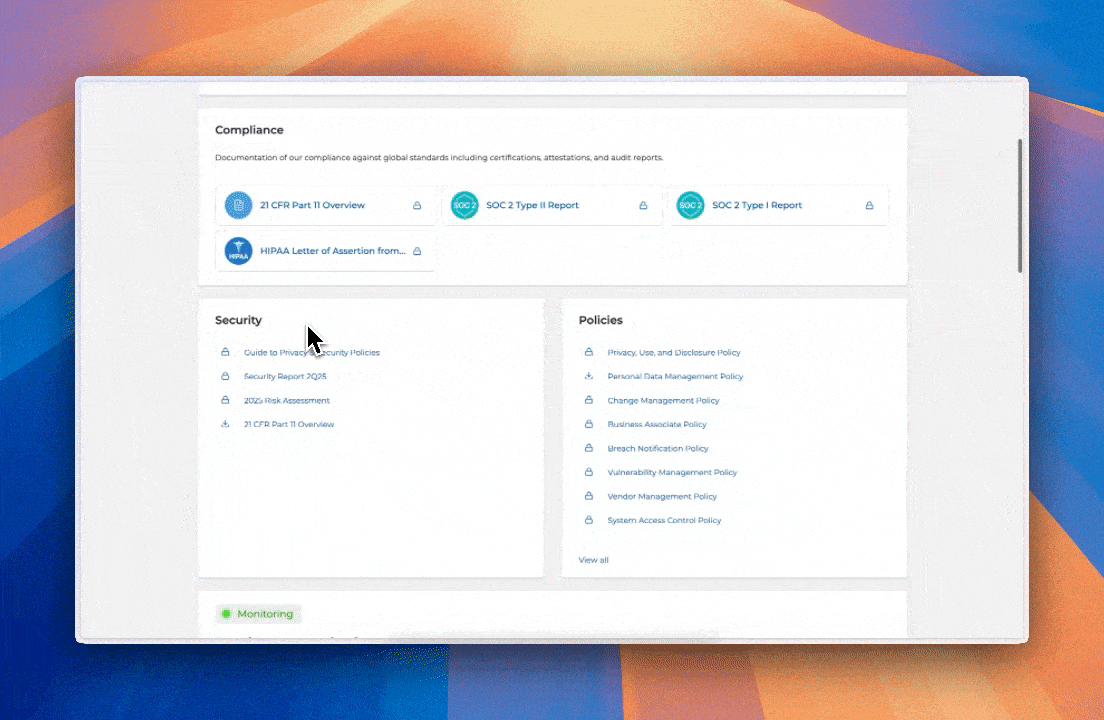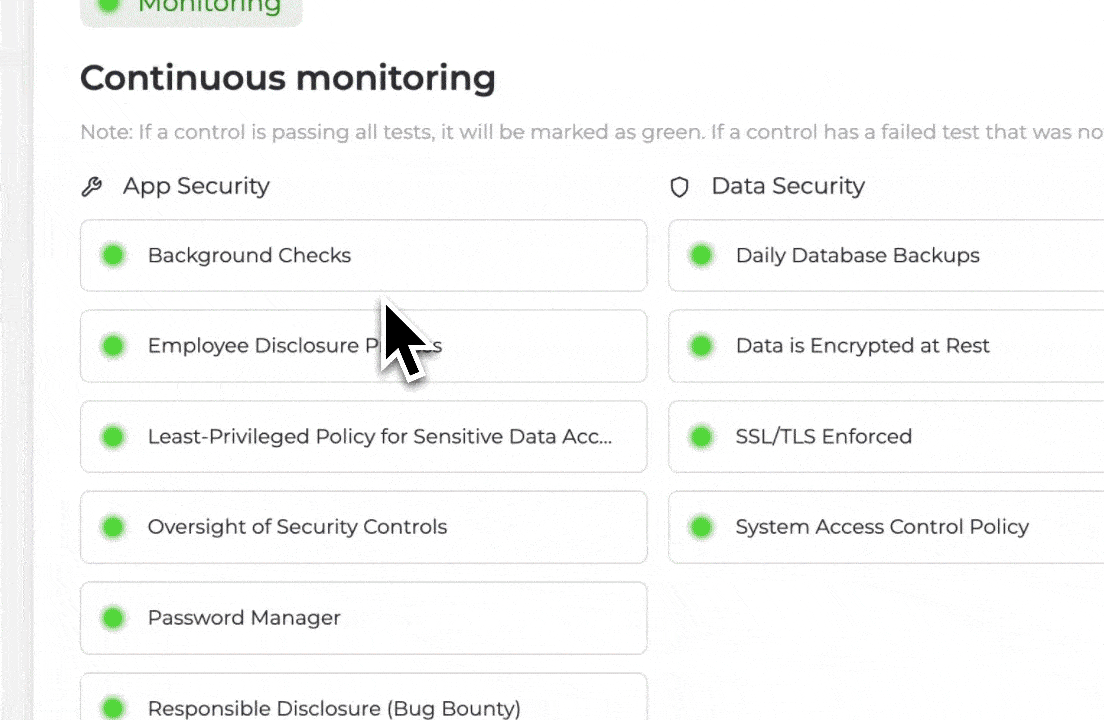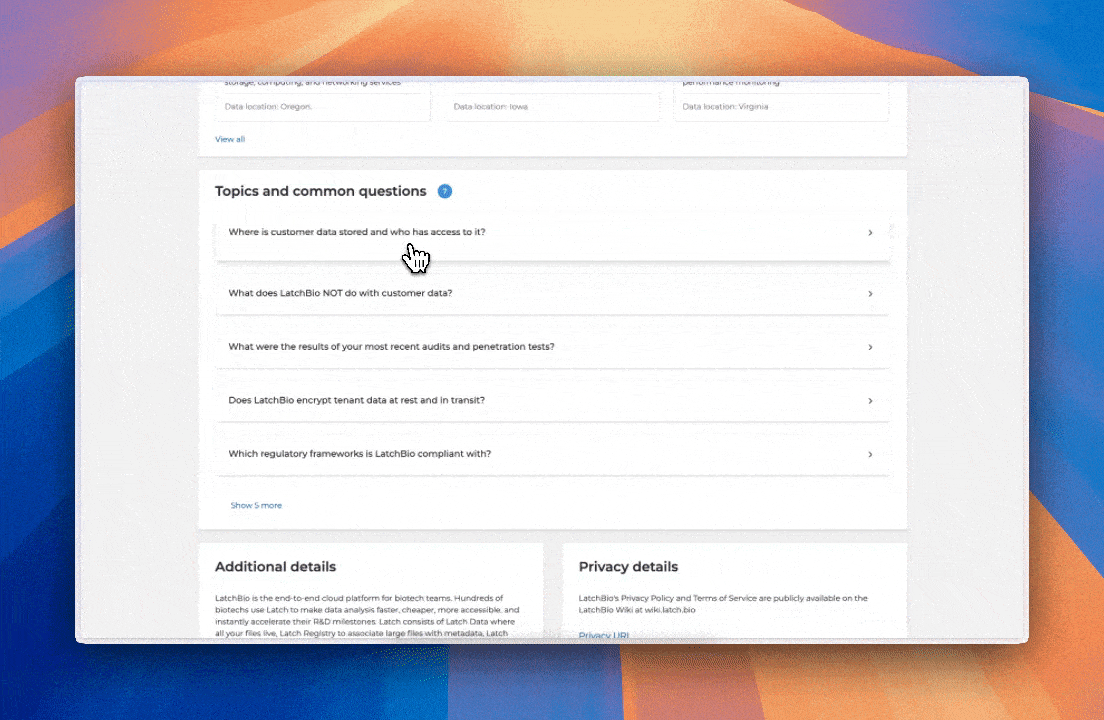
First, an Overview of Compliance at LatchBio
LatchBio’s Global Compliance Program is designed to optimize the speed of your discovery without sacrificing its security. We work closely with both technical and regulatory partners to ensure the highest level of compliance and confidence for our customers:
Drata provides 24/7 compliance monitoring visible to you,
NAMSA advises on biotech-specific regulatory positioning,
Tenax Solutions conducts annual penetration testing,
MJD Advisors audits the integrity of our commitments each year, and
GRCI Law employs our EU- and UK-based GDPR representatives and advises on international transfers.
Our aim is to meet every client’s regulatory requirements and work closely to provide regulatory support needed from a data processor hosting critical scientific infrastructure. To this end, we are compliant with a range of US and international frameworks, and quickly work with our customers when they need custom requirements or to comply with a different regulatory framework.
Latch is compliant with HIPAA, SOC 2, GxP, CLIA, CCPA/CPRA, and more. Visit our Trust Center to view our extensive security and compliance documentation.
Data Provenance and Reproducibility for Patient Rights
Across industries and countries, the public increasingly demands visibility into what companies know about them (e.g., requesting a copy of your personal data from Meta, or asking 23andMe to permanently delete your data). GDPR codified this cultural shift into law by giving patients clear rights to access, correct, or delete what companies know about them,2 including biotechs and solution providers.
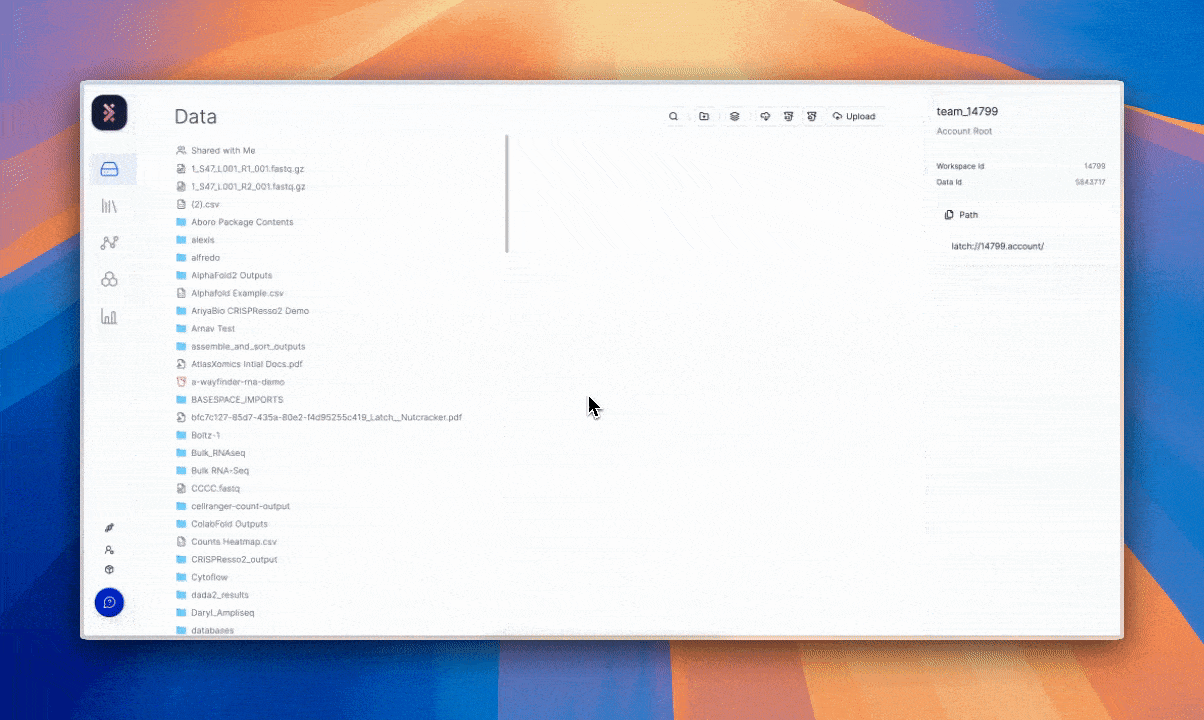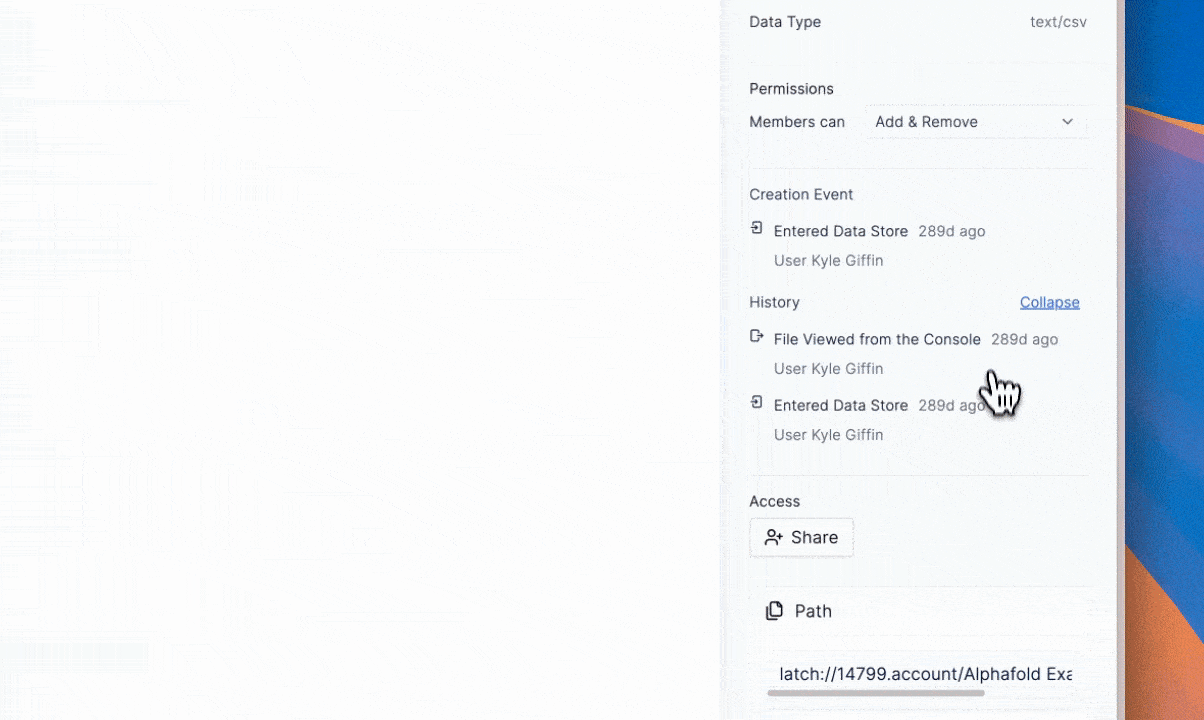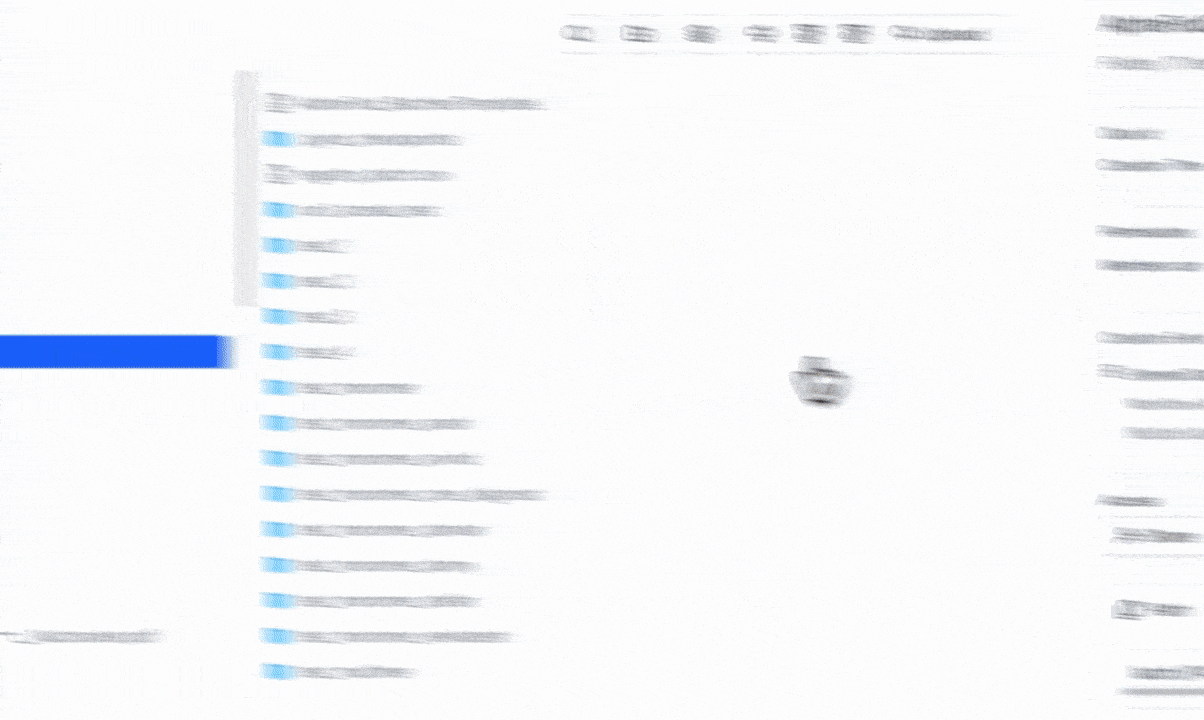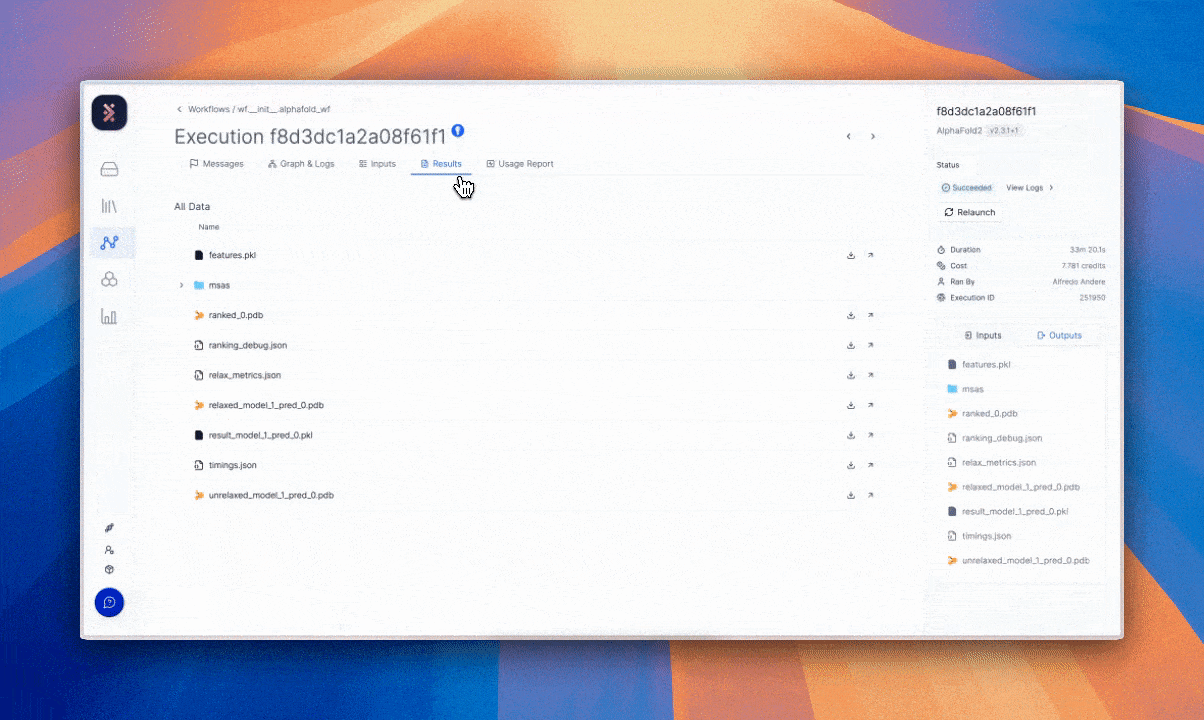
GDPR and complementary frameworks have requirements that are satisfied by immutable logs of processing history (e.g., every workflow, data transfer, analysis step, or single data manipulation),3 with evidence of who did what and when. If done poorly, this could result in a disarray of paperwork and messy file systems (think graduate student’s desktop with folders inside folders, labeled “final_v7_actually_final).”
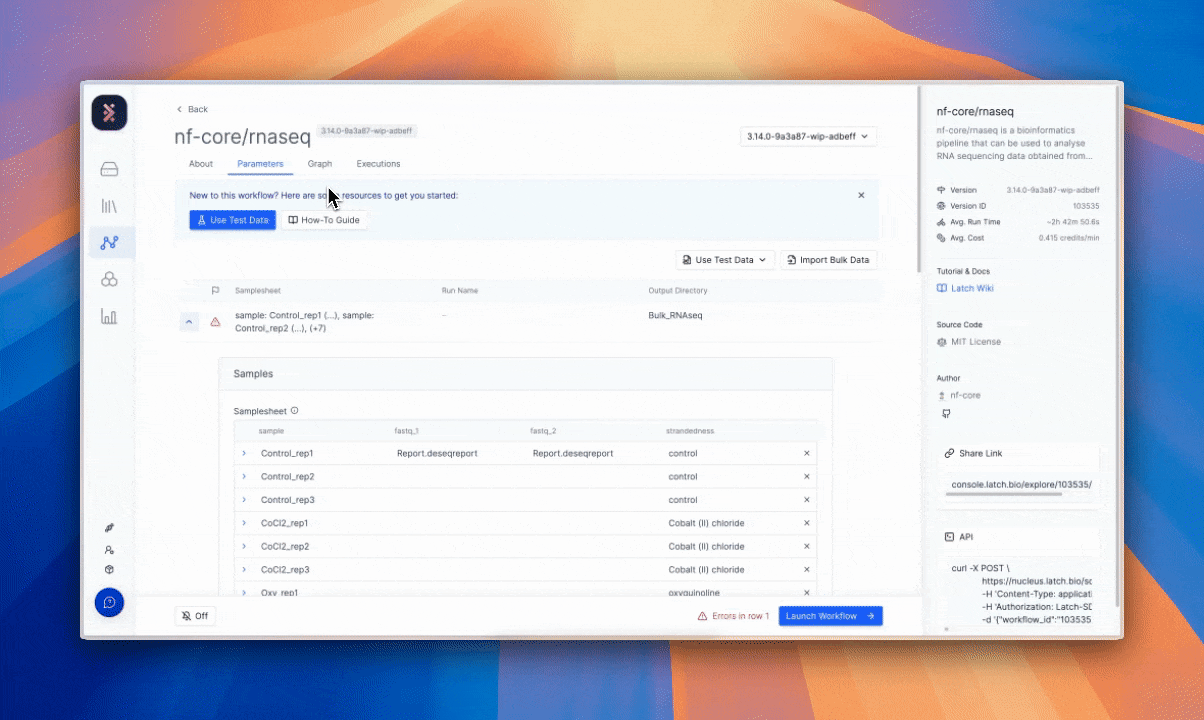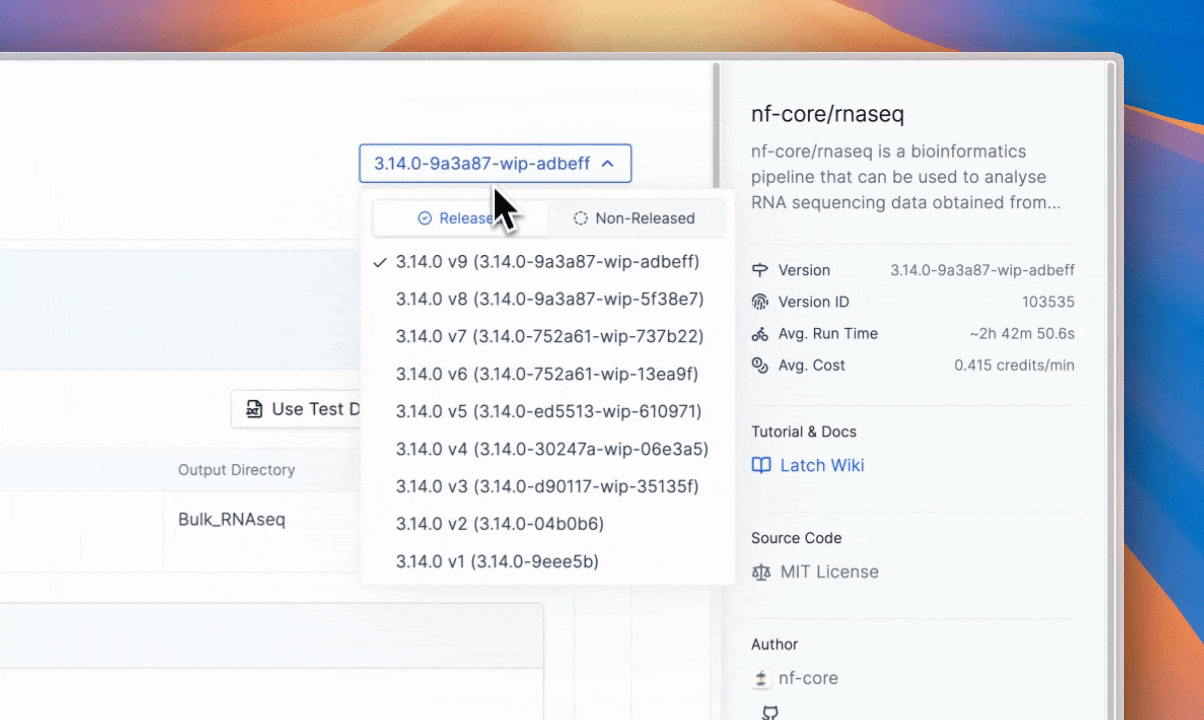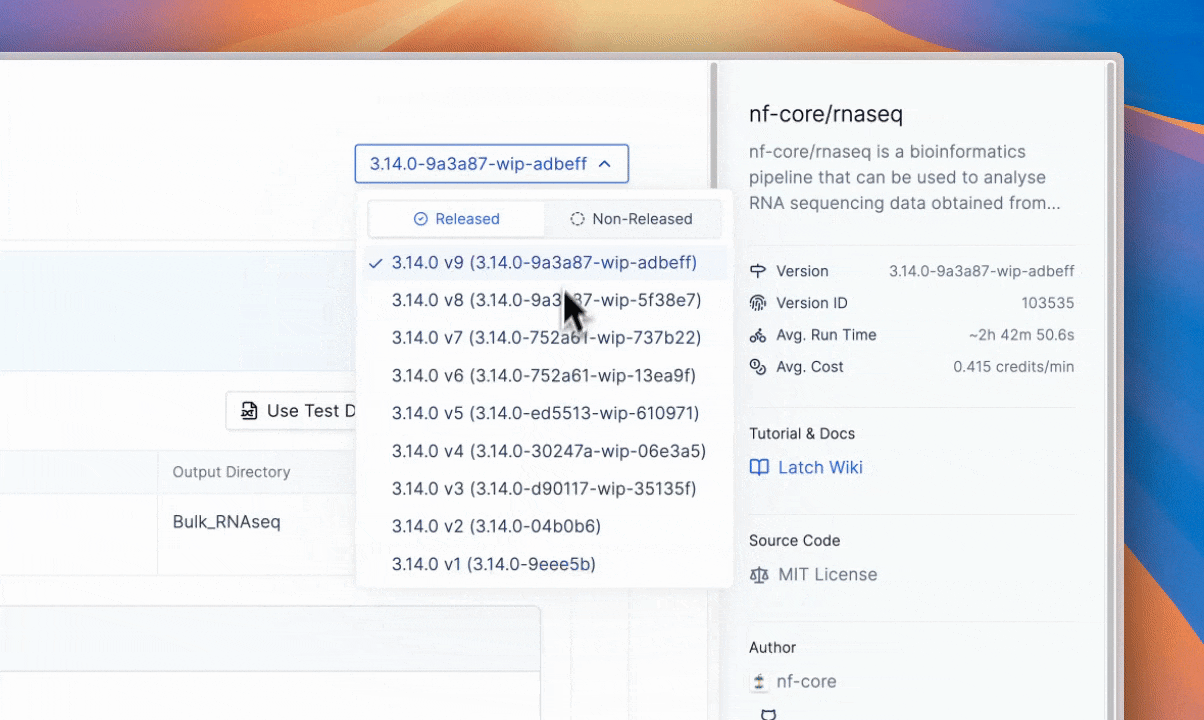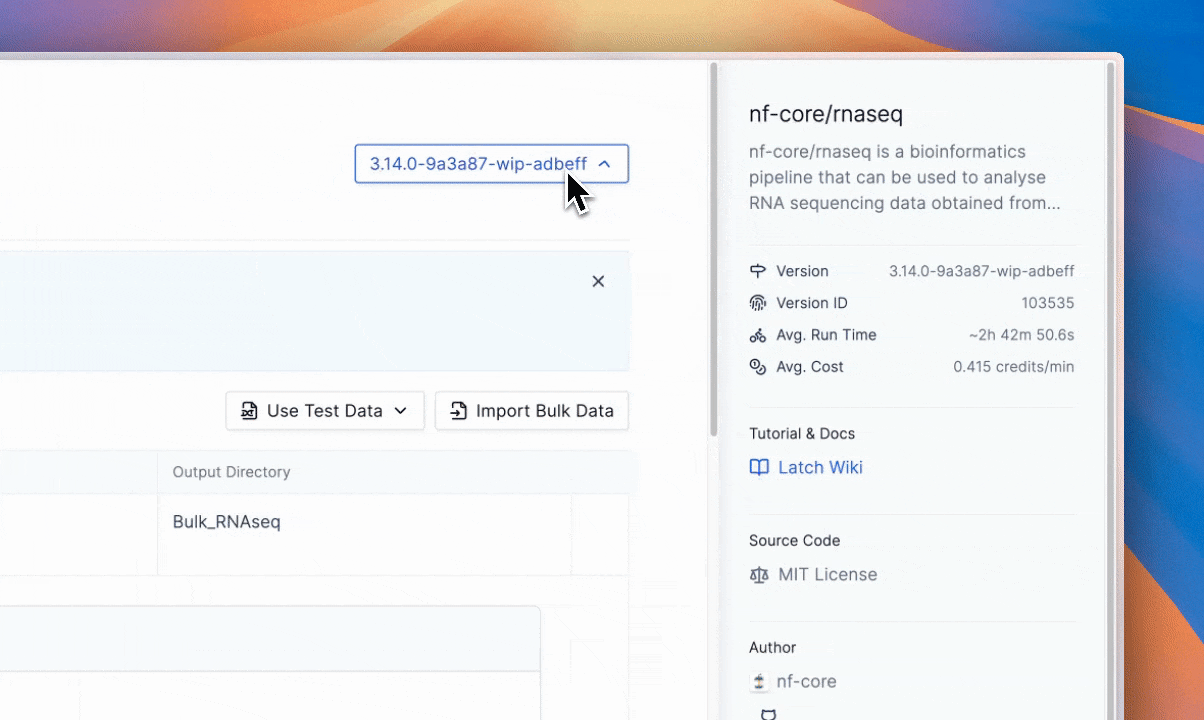
Latch’s architecture treats auditability and provenance as defaults. The full history of manipulation and record is available without manual reconstruction. The result is a system aligned with GDPR’s principles of accountability and transparency.4
The value of this requirement and solution can be interpreted beyond data subject rights. It allows scientific teams to reproduce an experiment exactly as it was run, returning to prior states and tracing how data evolved over time without guesswork to confirm the reliability of results.
Principle of Least Privilege in Modern Bioinformatics Management
Research data moves constantly, between collaborators, instruments, and workflows. Each transfer introduces potential for error: a file overwritten by someone who didn’t need editor access, a dataset manipulated without context, a result tied to the wrong version of an input. GDPR formalizes what lab operational excellence already demands — that access to data is deliberate, limited, and accountable.5
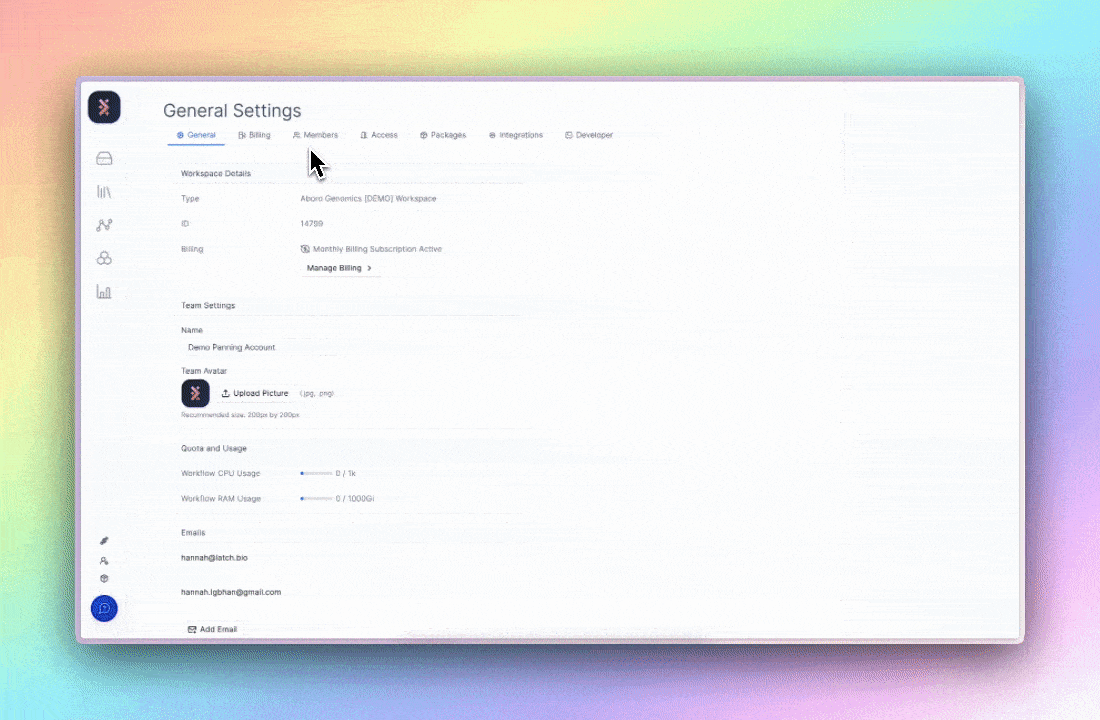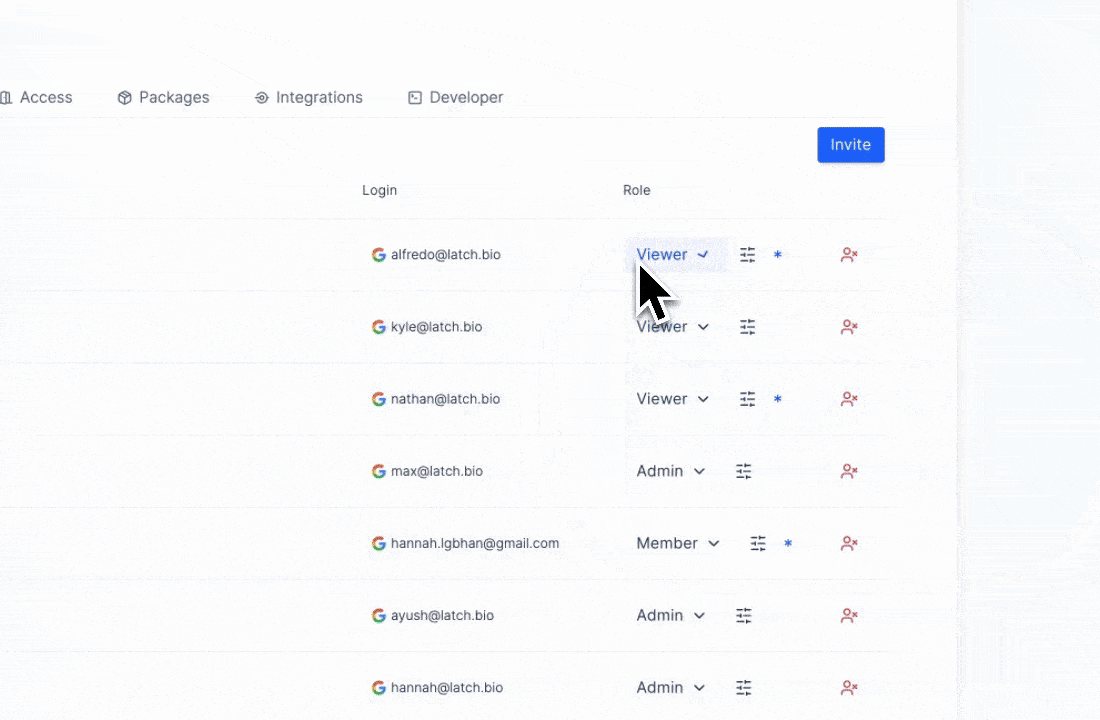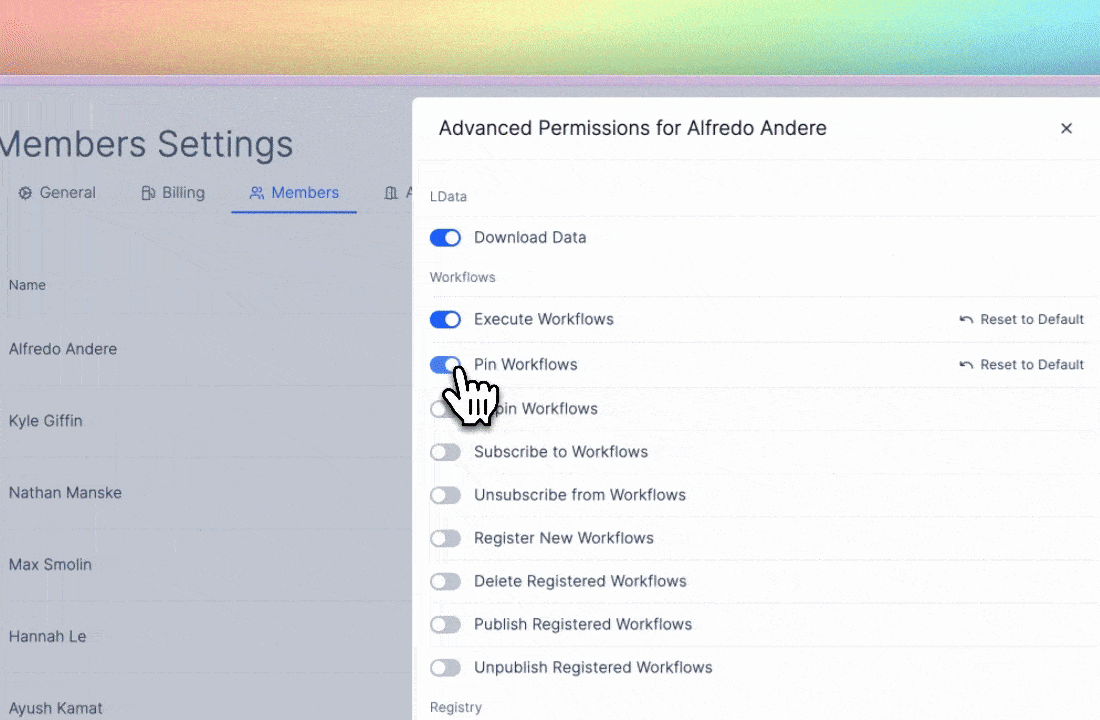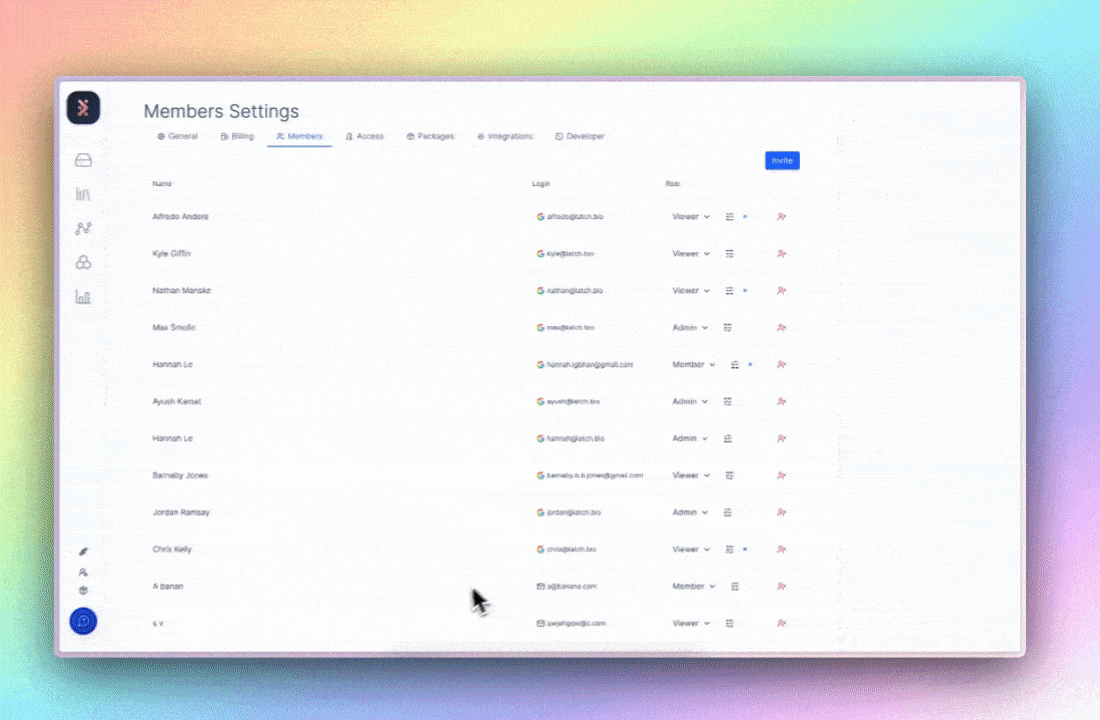
The principle of least privilege6 is, at its core, a safeguard against that kind of entropy. It requires admins to limit access to only what each user needs for their role and to keep detailed records of those interactions. In other words, it encodes good lab hygiene into law.
On Latch, this takes form through granular role-based access control (RBAC). Workspace owners define broad roles (viewer, editor, or admin) and then granularly customize permissions within those roles. Before high-impact actions like data export or deletion, user identity is re-verified7, and Latch engineers would have already been monitoring automatic signals for suspicious log-in behavior to report to admins.
The benefit isn’t just compliance; it’s coherence. Least-privilege access enforces experimental discipline: teams work from a single, trusted source, data integrity maintains intact, and the UI of each employee is specifically designated by the admin. What GDPR describes as “security of the processing” is, in practice, the same discipline required for reproducible, multi-stakeholder science.8
Commercial Advantage of GDPR Compliance for Biotech Solution Providers
In any pharma sale, the client’s procurement team will ask for a key set of GDPR-required artifacts, to size up a vendor’s readiness for regulated data transfer:
Standard Contractual Clauses (SCCs) define the terms for international data transfers9,
Data Processing Agreement (DPA) outlines how a processor manages and protects a controller’s data10,
Record of Processing Activities (ROPA) documents what data is handled, by whom, and for what purpose11,
Data Protection Impact Assessment (DPIA) evaluates risks in higher-impact processing, such as clinical or genomic data12,
Transfer Impact Assessment (TIA) assesses legal and technical safeguards for cross-border data13.
For solution providers, familiarity and preparation with these isn’t bureaucracy; it’s evidence of comprehensive risk management and sufficient understanding of complex international compliance. It shortens procurement cycles, and removes one of the most common blockers to large-scale deployments. Teams that can present these materials quickly are viewed as reliable partners who understand both the regulatory and operational realities of handling sensitive patient-derived data.
Latch integrates these same requirements directly into its onboarding and platform architecture. SCCs, DPAs, and supporting assessments are maintained and reviewed with independent auditors, creating a security and documentation layer that travels with your work. This foundation allows solution providers to engage with regulated customers without delay, building credibility while keeping the focus on scientific delivery rather than administrative process.
In Conclusion: Global Data, AI Models, and the New Relevance of GDPR
As science globalizes, GDPR’s reach extends naturally with it. Sequencing data, pathology images, and cellular atlases now cross continents daily to train AI models or power multi-omics pipelines. These datasets almost always contain information traceable to European individuals (directly or indirectly) which places them squarely under GDPR’s jurisdiction.
For solution providers, this means that data sovereignty and privacy controls are now core to scientific scalability. When you train an AI model on European datasets, deploy compute in the U.S., or serve results to a collaborator in Asia, GDPR follows the data at every step.
Far from being an obstacle, this consistency enables collaboration. Standardized privacy frameworks like GDPR create a shared security language between institutions, reducing friction in multinational research.14 They let scientists exchange data and model outputs with confidence that each participant is adhering to compatible privacy and accountability standards.
In this sense, GDPR has become part of the infrastructure of global bioinformatics: a framework that allows sensitive patient-derived data to move across borders, feed algorithms, and return as clinically meaningful insight, all while preserving the trust that underpins scientific progress.
If you are a solution provider interested in white-labeling these tools and offering them to customers through a branded portal, email contact@latch.bio.
LatchBio’s Compliance Team is available at compliance@latch.bio.
GDPR, Art. 83(5)
GDPR, Art. 15-17
GDPR, Art. 5(2), 30
GDPR, Art. 5(1)(a), 5(2)
GDPR, Art. 32
GDPR, Art. 32
Identity re-verification before manipulating data isn’t actually a GDPR requirement. It is a core tenet of the FDA’s 21 CFR Part. 11, but is similarly part of LatchBio’s internal design philosophy around RBAC protections.
GDPR, Art. 32(1)(d)
GDPR, Art. 46
GDPR, Art 28(3)
GDPR, Art. 30
GDPR, Art. 35
While not directly a requirement in GDPR, this requirement arose directly after an interpretation of GDPR Articles 44-46 in the landmark Court of Justice of the European Union’s Schrems II Ruling.
GDPR, Art. 44, 46